The Pulse — April 2025
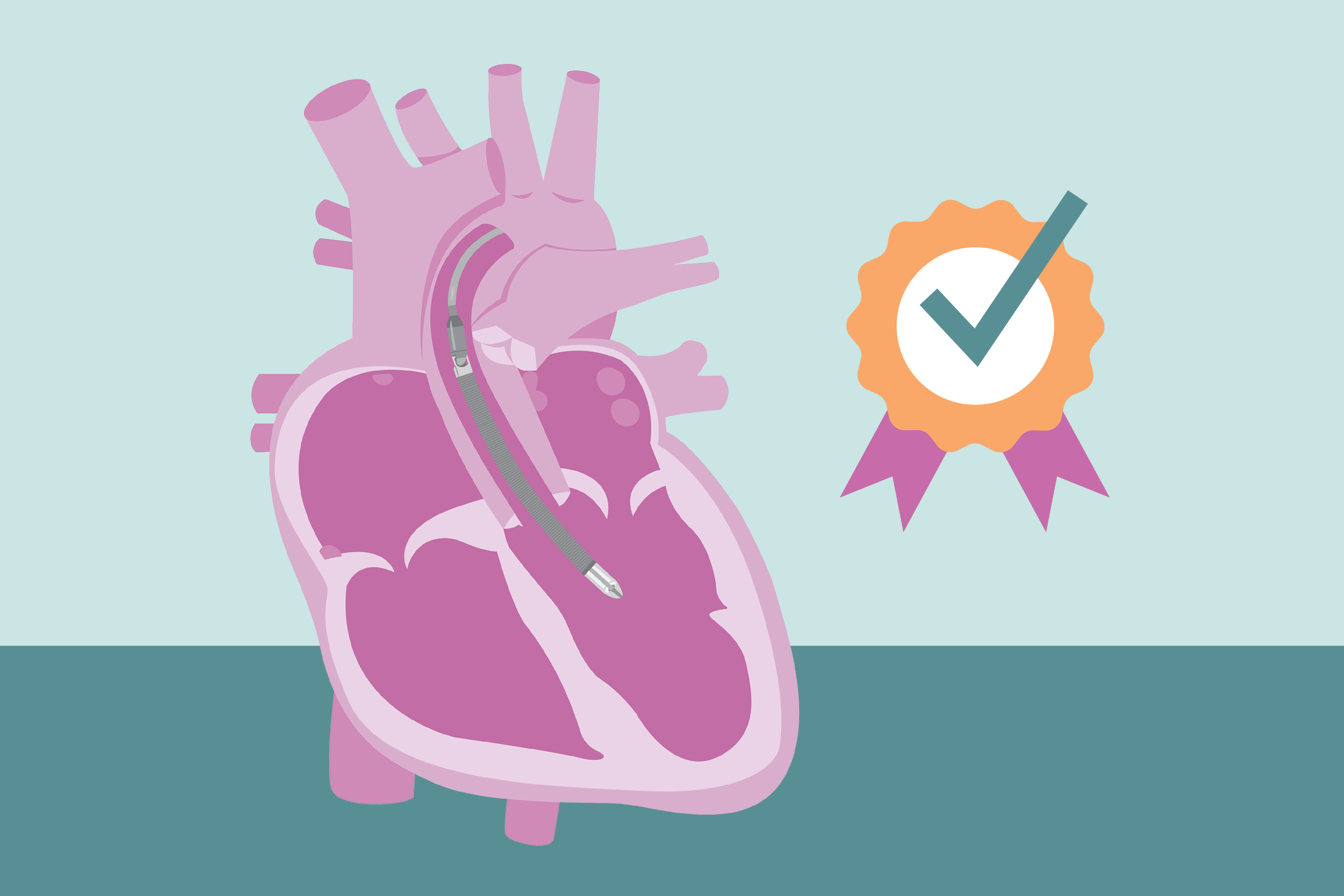
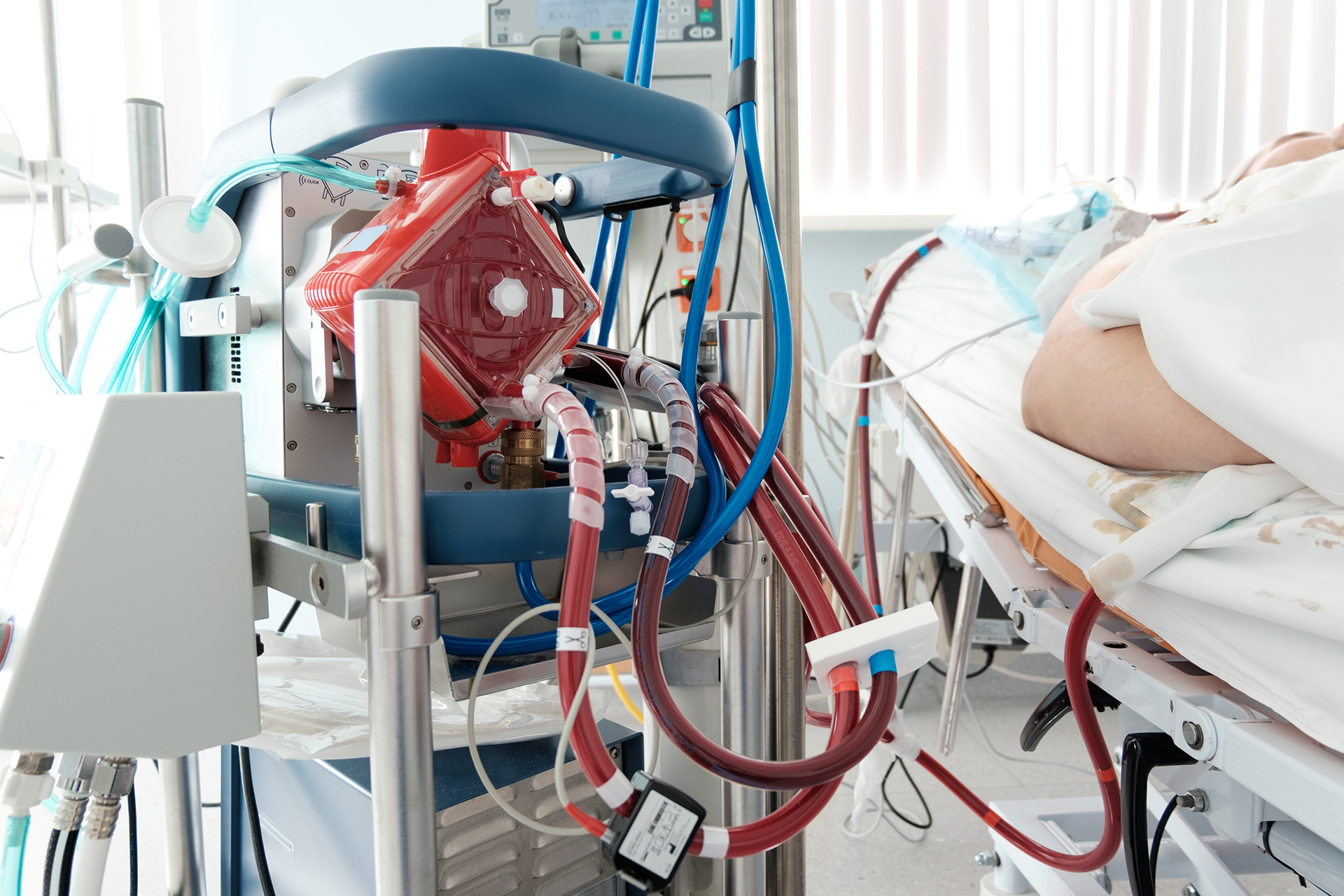
Let’s make 2025 our best year yet! We wrapped 2024 with the exciting announcement for the FDA expanded label of Johnson & Johnson MedTech’s Impella Support System to include treatment for certain children suffering from heart failure. Achievements like this would not be possible without the continued efforts and dedication of our ACTION centers providing care to these patients, as well as entering, cleaning, and adjudicating the data. We also continued access to the Berlin Heart Active Driver under the extended trial, and we published many new ACTION manuscripts.
The Pulse — April 2025 Read More »