Clinical Outcomes of SARS-CoV-2 Infection in Pediatric Patients on Ventricular Assist Device Support: An Action Registry Analysis
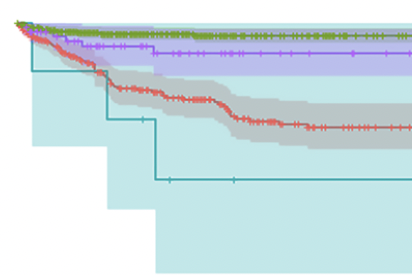
Abstract Adult patients on left ventricular assist device (LVAD) support have increased morbidity and mortality after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. There are no reported clinical data […]