Outcomes of Intracorporeal Continuous and Paracorporeal Pulsatile Ventricular Assist Devices in Pediatric Patients 10–30 kg
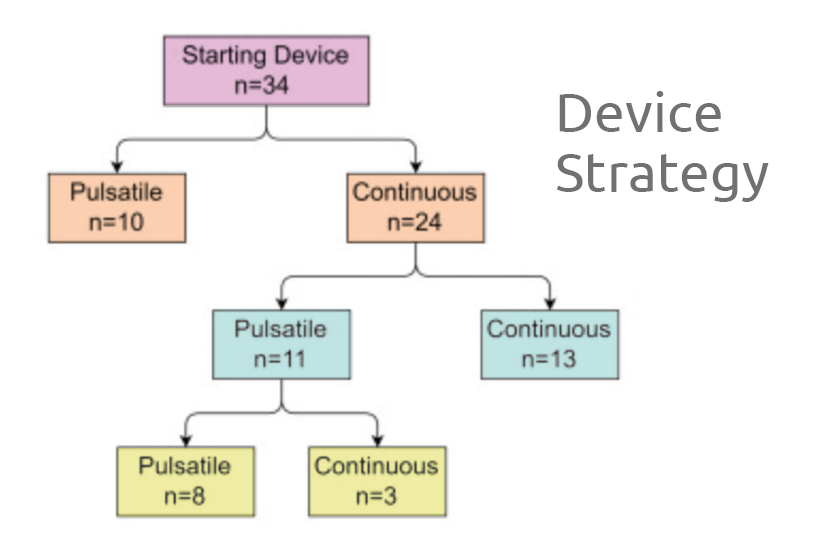
Ventricular assist devices (VADs) have been increasingly implanted in pediatric patients. Paracorporeal VADs are generally chosen when intracorporeal continuous (IC) devices are too large. Superiority between IC and paracorporeal pulsatile (PP) devices remains unclear in smaller pediatric patients. Our study analyzes outcomes of IC and PP VADs in pediatric patients who could be considered for either of these options.