IMPACT
Our Trials
Historically, there has been a paucity of pediatric device and drug trials due to cost, patient volume and variability in heart failure etiology. ACTION is collaborating with industry, patients and families and regulatory agencies to come up with novel ways to bring innovative care to the bedside. We are using the ACTION registry data as ‘real world data’ for both prospective clinical trials and to apply for expanded FDA indications for pediatrics.
The Berlin Heart EXCOR® Active Driver Trial
(sponsored by Berlin Heart, Inc., using the ACTION registry)


The Berlin Heart EXCOR® Active Driver Trial is sponsored by Berlin Heart, Inc. ACTION is serving as the clinical research organization (CRO) and the trial data is being collected through the ACTION registry.
The goal of the trial is to study the EXCOR® ACTIVE driver, which will be replacing the current IKUS (driving unit that powers the pump). The device/blood pump is still the same EXCOR® Pediatric VAD. Out of the 60 sites in ACTION, 15 sites have been chosen to be part of the trial.
See here for more information.
As of August 11, 2023

Patients Enrolled
40/40

Sites Enrolled
15/15
- Ann & Robert H Lurie Children’s Hospital
- Atrium Health Levine Children’s Hospital
- Boston Children’s Hospital
- Cincinnati Children’s
- Children’s Hospital Colorado
- Children’s Hospital Los Angeles
- Children’s Hospital of Philadelphia
- Childrens National Medical Center
- Children’s of Wisconsin
- Morgan Stanley Children’s Hospital of New York Presbyterian – Columbia
- Lucile Packard Children’s Hospital – Stanford
- Primary Children’s Hospital
- St. Louis Children’s – Washington University
- Texas Children’s Hospital
- UF Health Shands Children’s Hospital

Sites Activated
15/15
- Ann & Robert H Lurie Children’s Hospital
- Atrium Health Levine Children’s Hospital
- Boston Children’s Hospital
- Cincinnati Children’s
- Children’s Hospital Colorado
- Children’s Hospital Los Angeles
- Children’s Hospital of Philadelphia
- Childrens National Medical Center
- Children’s of Wisconsin
- Morgan Stanley Children’s Hospital of New York Presbyterian – Columbia
- Lucile Packard Children’s Hospital – Stanford
- Primary Children’s Hospital
- St. Louis Children’s – Washington University
- Texas Children’s Hospital
- UF Health Shands Children’s Hospital
The HeartMate 3TM VAD Expanded Label
(sponsored by Abbott, using the ACTION registry)

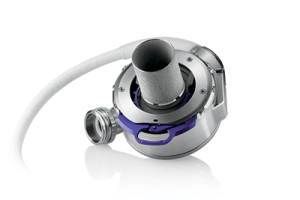
The HeartMate 3TM VAD was used in pediatrics for compassionate use as it was not approved for children, leading to a lack of pediatric training and pediatric specific educational materials. ACTION collaborated with Abbott to collect and adjudicate safety and efficacy data from 20 sites. The data was used for an application to the FDA, and in December 2020, the HeartMate 3TM VAD received an expanded label to include children. After the indication was broadened, pediatric educational materials were co-created through a partnership between Abbott and ACTION. ACTION is currently collecting the post surveillance data.

